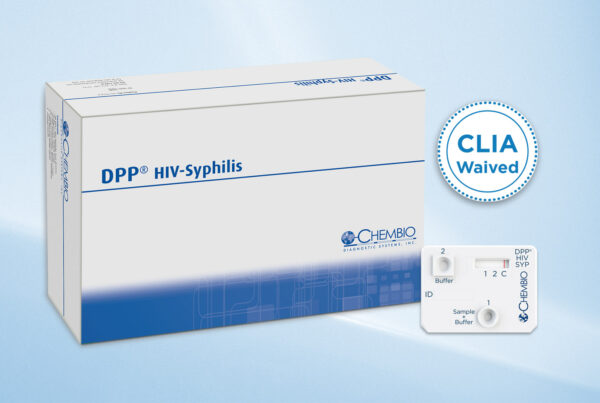
Medford N.Y USA/Strasbourg France – June 17, 2025 – Chembio Diagnostic Systems, Inc., a subsidiary of Biosynex, developer and manufacturer of rapid diagnostic tests, announced today that the U.S. Food…
Chembio Diagnostics Inc., a subsidiary of Biosynex, receives a grant for Development of a DPP Congenital Syphilis Assay Medford N.Y USA/Strasbourg France , Oct 23, 2024 Chembio Diagnostics, Inc. (Chembio), a…

Chembio Diagnostics, Inc. becomes the first North American structure controlled by Biosynex in North America STRASBOURG, France, April 27, 2023 (GLOBE NEWSWIRE) -- Biosynex SA (“Biosynex”) (EPA: ALBIO), a French market…