A 15 minute rapid test for detection of Ebola Virus Specific Antigen
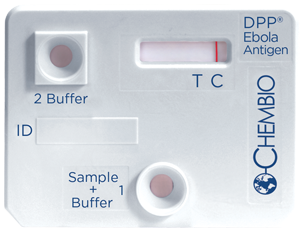
For the detection of antigen specific to Ebola Virus in fingerstick whole blood, EDTA venous whole blood and EDTA plasma.
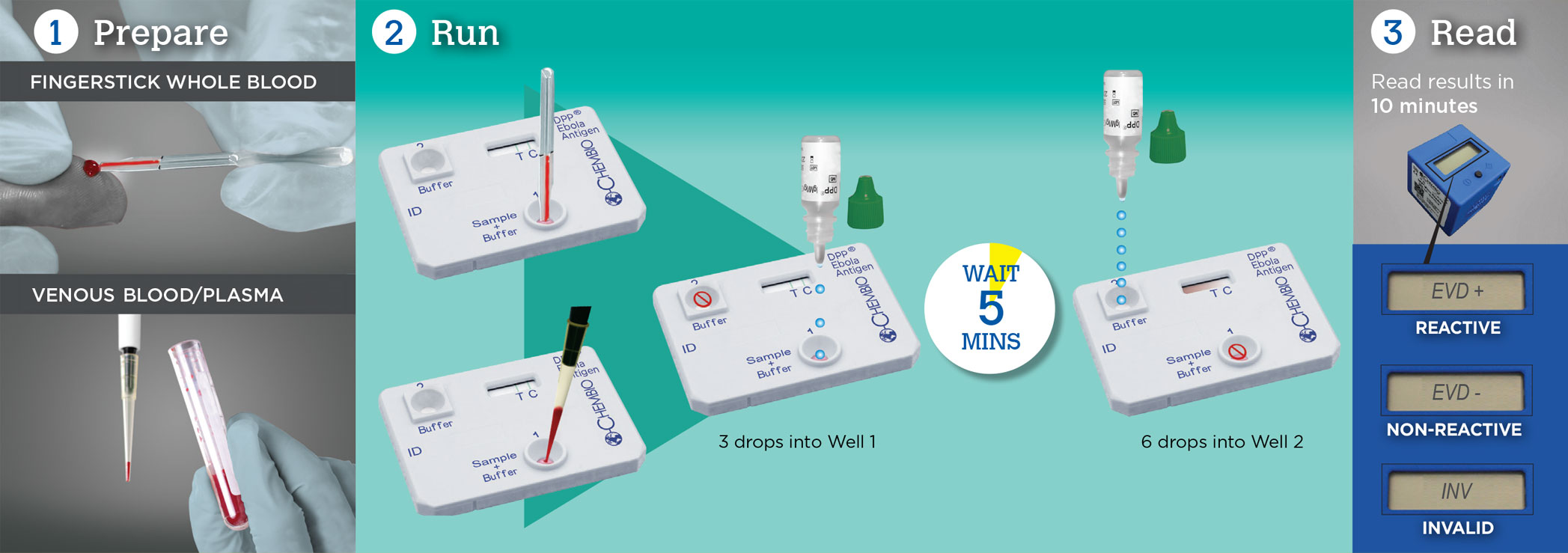
Test for Ebola in 3 Easy Steps with DPP®
Product Downloads & Links
Please fill out the form below to receive the following downloads and links:
- EUA Authorization Letter
- Product Insert
- Fact Sheet for Health Care Workers
- Fact Sheet for Patients
Ordering Information
| Product | Catalog Number | |
| DPP® Ebola Antigen System | 61-1013-0 | |
| DPP® Micro Reader for usewith the DPP® Ebola Antigen System | 61-1050-0 | |
| DPP® Ebola Rapid Test Control Pack | 60-9554-0 | |



