For the detection of Zika virus IgM antibodies in fingerstick whole blood, EDTA venous whole blood, EDTA plasma and serum.
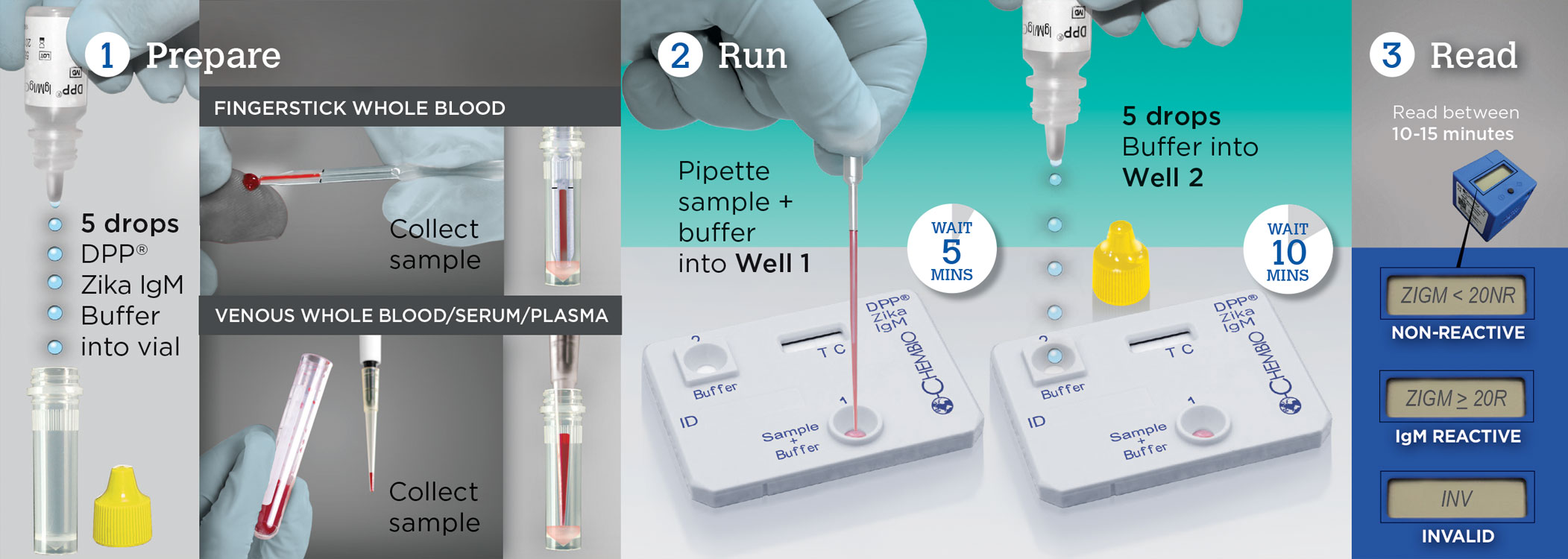
Test for Zika in 3 Easy Steps with DPP® Zika IgM System
DPP® Zika IgM System Using Fingerstick Blood
This video will show you how to perform the test using Fingerstick Blood. The DPP® Zika IgM System is a 15 minute rapid test for detecting Zika virus infection.
How to Use The DPP Zika IgM System Using Venous Whole Blood, Serum & Plasma
This video will show you how to perform the test using Venous Whole Blood. The DPP® Zika IgM System is a 15 minute rapid test for detecting Zika virus infection.
Product Performance
| Sample Type | Positive Percent Agreement | Negative Percent Agreement | ||
| Venous Whole Blood* | 99.4% (178/179) | 100% (120/120) | ||
*Refer to product insert for more details.
Product Downloads & Links
Please fill out the form below to receive the following downloads and links:
- Product Insert
- Quick Reference Instructions
Ordering Information
| Product | Catalog Number | |
| DPP® Zika IgM Assay System | 65-9560-0 | |
| DPP® Zika IgM Micro Reader | 70-1064-0 | |
| DPP® Zika IgM Control Pack | 62-1001-1 | |