Contact your local Chembio Representative to learn more about our Detect test.
An FDA EUA visual read rapid self-test for detection of SARS-CoV-2 Antigen in nasal swab specimens.
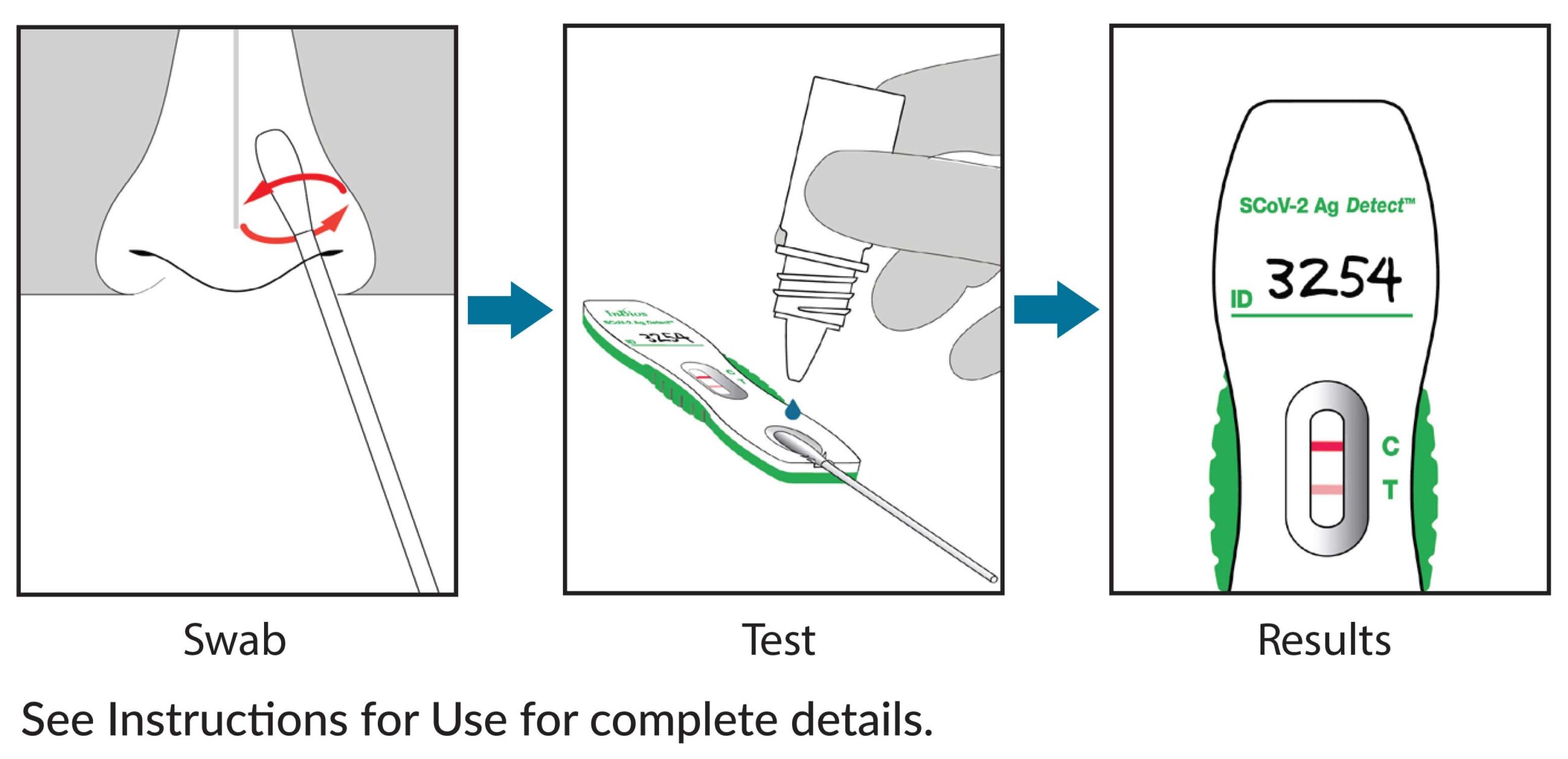
Test for SARS-CoV-2 in 3 Easy Steps with SCoV-2 Ag Detect™ Rapid Self-test*
Chembio has been an innovator in infectious disease testing for over 25 years with specific expertise in HIV, Syphilis, Zika, Ebola, Dengue, Chikungunya and others.
Product Information*
| Information Type | Product Details |
| Time to Results | 20 minutes |
| Sample | Minimally invasive shallow nasal swab specimen |
| Result Interpretation | Visual Read; No instrument required |
| Storage Conditions | Room Temperature |
| Method | Lateral Flow |
* Refer to product insert for details
Ordering Information
| Product | # of Tests | Catalog Number |
| SCoV-2 Ag Detect™ Rapid Self Test | 2 | CAGS-2-CDS |
* This test has not been FDA cleared or approved, but has been authorized by FDA under an EUA for use by authorized laboratories; This test has been authorized only for the detection ofproteins from SARSCoV-2, influenza A and influenza B, not for any other viruses or pathogens; The emergency use of this test is only authorized for the duration of the declaration thatcircumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug andCosmetic Act, 21 U.S.C. 360bbb-3(b)(1), unless the declaration is terminated or authorization is revoked sooner.