Contact your local Chembio Representative to learn more about our Status test.
Status Flu A & B is a CLIA Waived in vitro rapid qualitative test that detects Influenza Types A and B antigens directly from nasal or nasopharyngeal swab specimens.
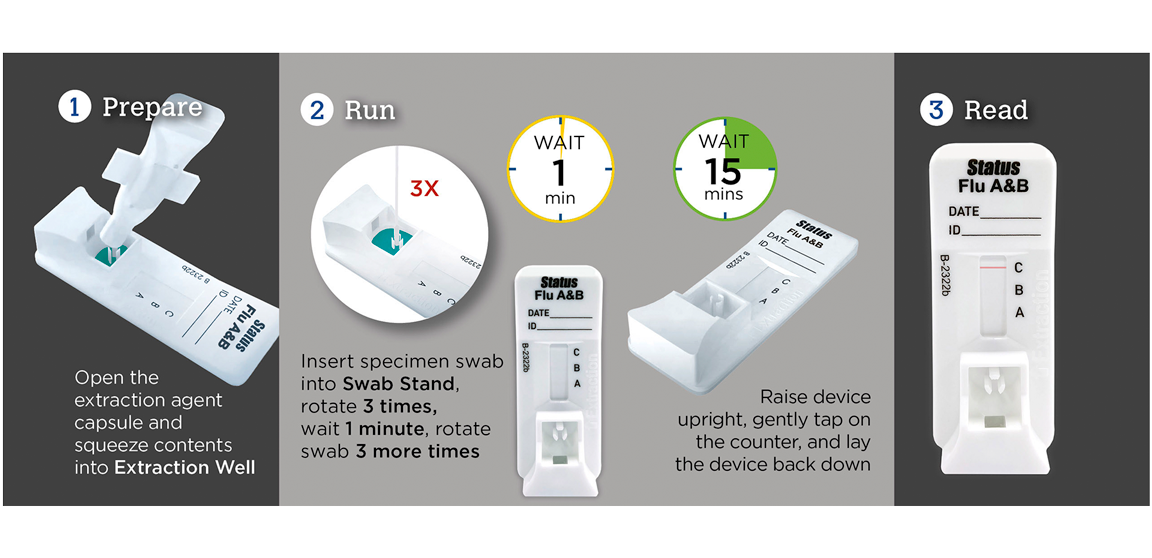
Test for Influenza Types A & B in Just a Few Simple Steps
Product Information**
| Information Type | Product Details |
| Time to Results | 10 - 15 minutes |
| Sample | Nasal or Nasopharyngeal Swab Specimen |
| Result Interpretation | Visual Read |
| Storage Conditions | 36˚ to 86˚ F (2˚to 30˚ C) |
| Method | Lateral Flow |
| CPT Codes | 87804-QW, 87804-QW-59 More Reimbursement Information |
Ordering Information
| Product | # of Tests | Catalog Number |
| Status® Flu A & B | 25 | 36025 |
| Control Kit | 5 | 36022CS |






